
Top Performing Drug of 2021 - Trulicity (February Edition)
Active Ingredient: dulaglutide
Dosage Forms & Strengths:
Injection: 0.75 mg/0.5 mL, 1.5 mg/0.5 mL, 3 mg/0.5 mL, 4.5 mg/0.5 mL solution in a single-dose pen
Mechanism of Action: GLP-1 receptor agonist
First Approval: US (Sep 18, 2014), EU (Nov 25, 2014)
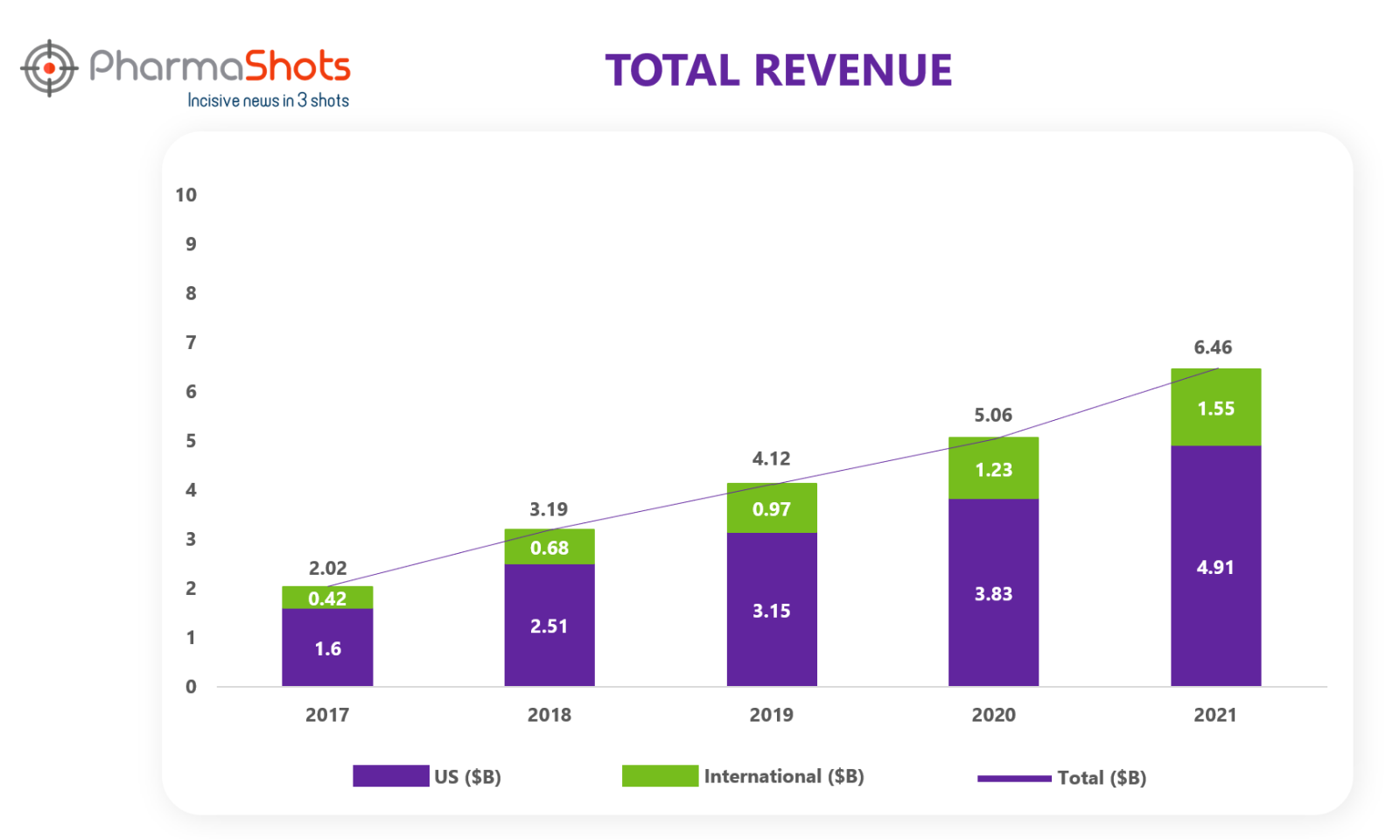
Revenue Analysis1
Trulicity is used for the treatment of type 2 diabetes and to reduce the risk of major adverse cardiovascular events in adult patients with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors. It is one of the key growth products of Eli Lilly. In 2021, the revenue of Trulicity increased by 28% in the U.S., driven by increased demand, and by 26% outside the US.
If we look at the past 5 years’ revenue analysis of Trulicity, we can see rapid growth of the product in the past years.
Approved Indications of Trulicity2
Trulicity is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated:
- As an adjunct to diet and exercise to improve glycaemic control in adults and paediatric patients (age: ≥10 years) with type 2 diabetes mellitus.
- To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors.
Limitations of Use:
Certain limitations for the use of Trulicity include:
- Other antidiabetic therapies are recommended for patients with a history of pancreatitis as Trulicity has not been studied in these patients
- It is not used for the treatment of patients with type 1 diabetes mellitus
- It is not recommended in patients with severe gastrointestinal disease, including severe gastroparesis
Clinical Trials Analysis3
Trulicity has a total of 104 trials, incl. 71 industry trials of which 68 are interventional and 3 are observational. The analysis of industry trials through a representation is shown below (Trials are taken as of 7 Feb 2023).
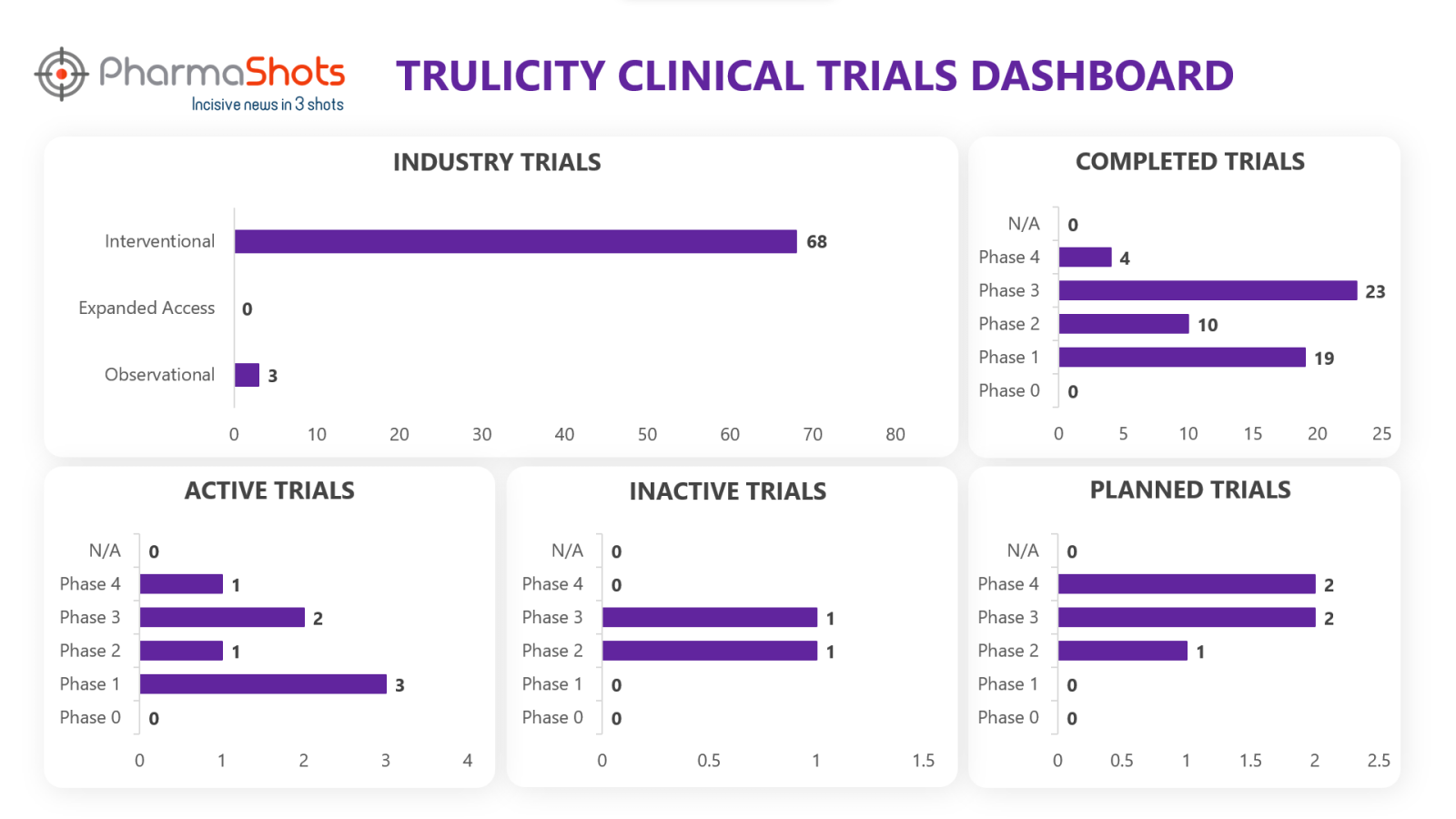
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
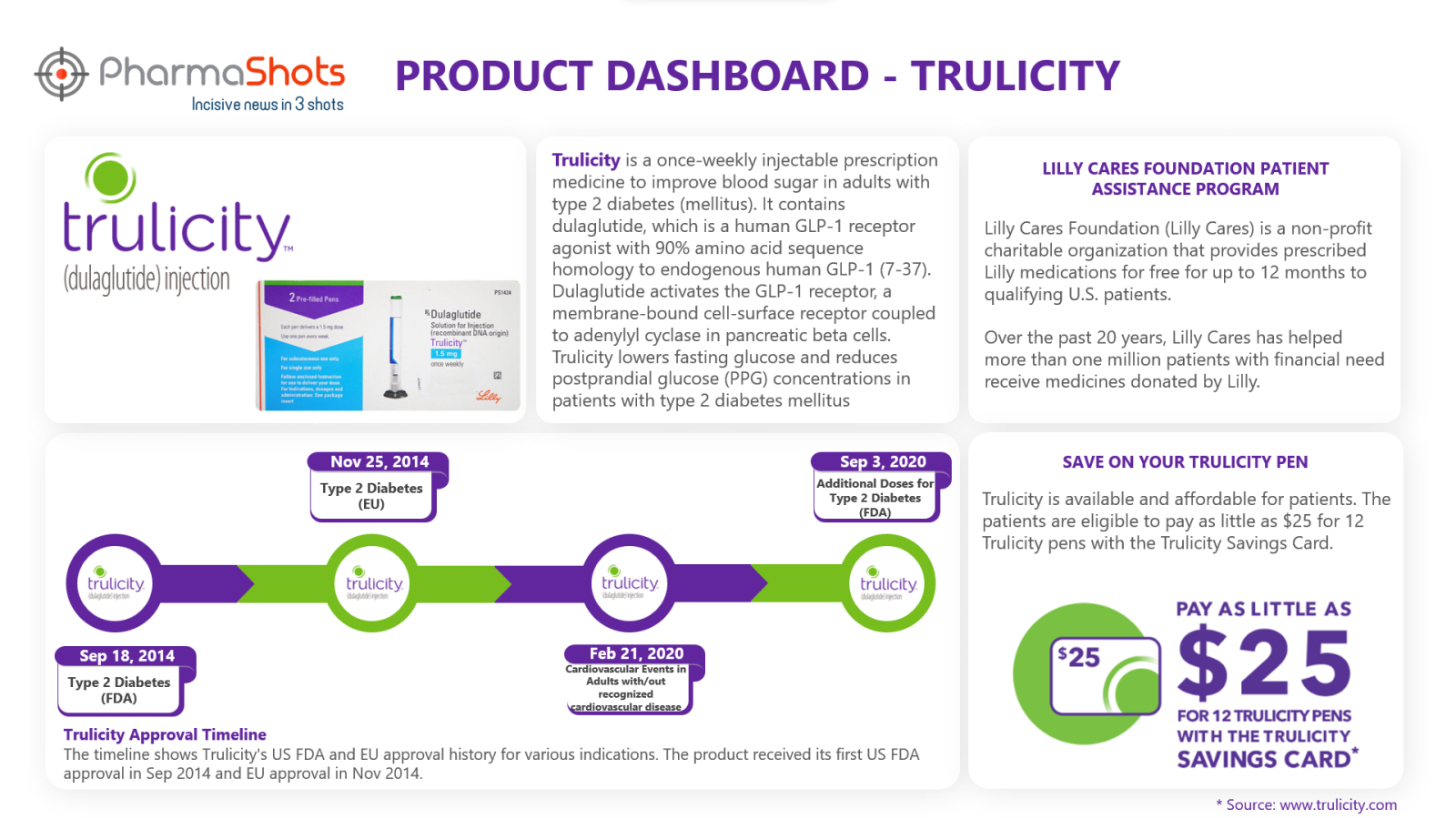
Lilly Cares Foundation Patient Assistance Program4
This advocacy program can help patients with financial needs receive their prescribed Eli Lilly medications at no cost. The program is run by Lilly Cares Foundation (Lilly Cares) a non-profit charitable organization that provides prescribed Lilly medications for free for up to 12 months to qualifying U.S. patients.
Over the past 20 years, Lilly Cares has helped more than one million patients with financial needs receive medicines donated by Lilly.
The program covers various medications of Lilly including Trulicity. The patients are eligible for the Lilly Cares program if:
- They are a permanent, legal resident of the United States, Puerto Rico, or the U.S. Virgin Islands.
- A qualifying medication is prescribed by their healthcare provider
- The patients who have no insurance or have Medicare Part D
- Patients who are not enrolled in Medicaid, full Low-Income Subsidy, or Veterans (VA) benefits.
- Those household income guidelines for the program.
The program has divided the different medications into 3 Groups depending on gross income. These are divided as:
- Group 1 Medications: For patients who have no insurance or have Medicare Part D and have a household annual adjusted gross income ≤300% Federal Poverty Level (FPL).
- Group 2 Medications: For patients who have no insurance or have Medicare Part D and have a household annual adjusted gross income ≤400% FPL.
- Group 3 Medications: For patients who have no insurance, or have Medicare Part D and have a household annual adjusted gross income ≤500% FPL.
*Trulicity is covered under the Group 2 medications
How to Apply?
- Patients can fill out an online application form. The application form is also available in the Spanish version. The form can also be mailed to the patients by calling on 1-800-545-6962.
- The patient needs to fill out and sign the application form. The prescriber section and the prescription need to be filled by the healthcare provider of the patients.
- After the submission, Lilly Cares will review the application and provide patients and their healthcare provider with an enrollment decision through mail or text.
- If the application is approved, the enrollment notification letter will be given which will contain the expiry date.
- After that, the medications will either be shipped to the patient’s home or to the healthcare provider’s office.
- The patients can reapply for the program at the end of the enrollment period to remain eligible for the program
Save on your Trulicity Pen5
A Trulicity Savings Card is developed to help patients with diabetes so that they can afford their medication. If eligible, they may pay as little as $25 for 12 Trulicity pens. The patient must-have commercial health insurance to use the savings card. Those with Medicare or Medicaid coverage are not eligible.
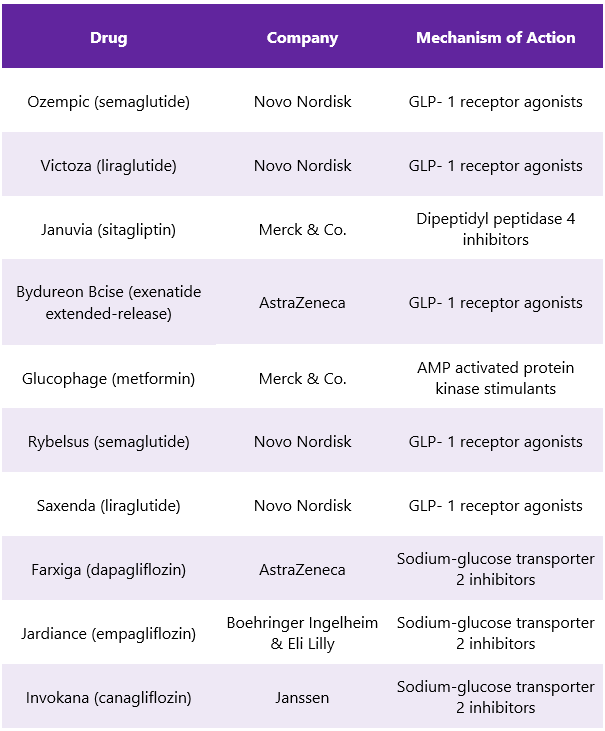
Trulicity Competitors6
There are many other alternative types of diabetes medications present in the market that gives tough opposition to Trulicity. Some of its FDA-approved competitors include:
Patents7
Eli Lilly carries big expectations from Trulicity to boost its revenue. Hence, it needs to protect its patent as Trulicity patents are very vital to the company’s business as a whole. In the US, Trulicity is protected by a compound patent (2027) and by biologics data protection (2026). Outside the US it is protected by:
- a compound patent (2029)
- data protection (2024) in major European countries
- a compound patent (2029) and by data protection (2023) in Japan
Trulicity Patient Stories8
Patient stories are beneficial in addressing things like communication difficulties, that are directly connected to the patient's impression of care. Trulicity has been a part of over 2.8 million patients’ journeys with type 2 diabetes since 2014.
TruStories gives the opportunity to meet some of these patients, as well as their loved ones, and hear how Trulicity along with diet and exercise helped control their A1C. Below mentioned are some of the cases:
1. Story of Chuck
Chuck is a Trulicity patient. He told about the important lifestyle changes he has made and how once-weekly dosing with Trulicity fits into his schedule. Chuck said, “I hope my Trulicity story is helpful by providing additional information about my experience.”
2. Story of Wylena
Wylena is a Trulicity patient. Wylena’s shared her experience of how important was the diagnosis and treatment to them, and how Trulicity has helped with type 2 diabetes. She said, “I wanted to share my story so others can know more about Trulicity.”
3. Story of Cesar
Cesar is a Trulicity patient who worked as a registered nurse for over 20 years. Cesar talks about the role Trulicity played, along with diet and exercise, helping him to reach his A1C goals and how he wishes to pass on his learnings for healthier habits to his growing family. He said, “I hope my Trulicity story and experience can be a source of inspiration for others.”
References:
- Eli Lilly 10-K Reports
- Trulicity Prescribing Information
- clinicaltrials.gov
- Lilly Cares Foundation Patient Assistance Program
- Save on your Trulicity Pen
- Trulicity Competitors
- Patents
- Trulicity Patient Stories
Related Post: Top Performing Drug of 2021 – Imbruvica (January Edition)
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.














